Medical device adhesives are critical components, especially in implantable devices, where reliability is paramount. Medical device adhesives must bond reliably. Preventing device failure or poor performance is why these products must meet strict internal, Federal, and international standards, like ISO 9001, ISO 13485, and ISO 14001. Despite meeting these standards, adhesives can experience a decline in bonding strength over time if not properly cured.
Key Points:
1. Adhesive Curing Methodology
The choice of curing method significantly impacts the bonding strength and overall performance of medical device adhesives. Factors such as bond strength requirements, presence of bubble voids, compatibility with sterilization methods, and physical properties of the parts influence the selection process. For instance, certain adhesives may require light curing for rapid activation, while others may benefit more from thermal curing to achieve optimal bonding.
The table below shows the critical considerations when choosing a curing method:
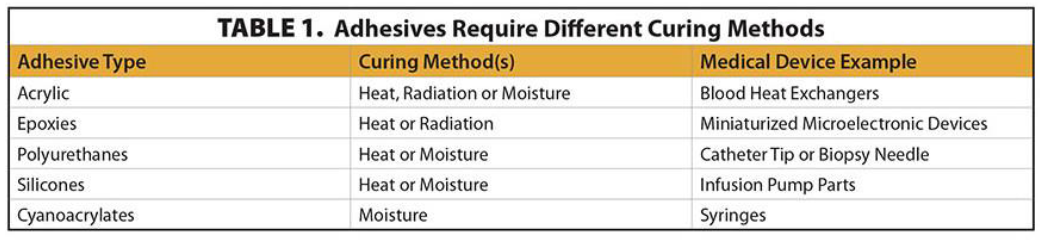
2. Light Curing Considerations
Light curing, utilizing photo initiators activated by specific wavelengths of light, offers advantages such as rapid curing times and suitability for automated assembly processes. However, it is essential to address potential issues such as the formation of air bubbles within the adhesive during curing. Understanding the limitations of light curing is crucial for mitigating these challenges and optimizing bonding strength.
3. Importance of Thermal Curing
Thermal curing isn’t going away any time soon. It’s still a critical step in many medical device manufacturing applications. Beyond ensuring repeatability, thermal curing enhances adhesive properties such as hardness, rigidity, and impact resistance. Manufacturers can maintain ISO 5 cleanroom conditions throughout the curing process, further ensuring the quality and reliability of medical device adhesives.
4. Addressing Long Cure Times
Many thermally cured adhesives cure at room temperatures. But that cure time is often too long for many medical applications. Long cure times increase work-in-progress, boost production costs, and foster poor quality. Manufacturers may explore curing at higher temperatures to expedite the process, provided that the substrate and adhesive can withstand such conditions. Collaboration with adhesive suppliers and coating manufacturers is essential to determine the minimum cure times required for specific temperatures, optimizing efficiency without compromising bonding strength.
5. Critical Role of Curing in Life-Saving Devices
Some medical device applications require multiple curing steps. Each step is unique. Each also has its own set of constraints, materials, geometries, and functions. When manufacturing life-saving devices, which often have a high risk of failure, manufacturers must qualify the production processes. That ensures these devices perform as expected and meet all requirements. Medical device manufacturers often use thermally cured adhesives for these critical curing steps because of their reliability and reliability.
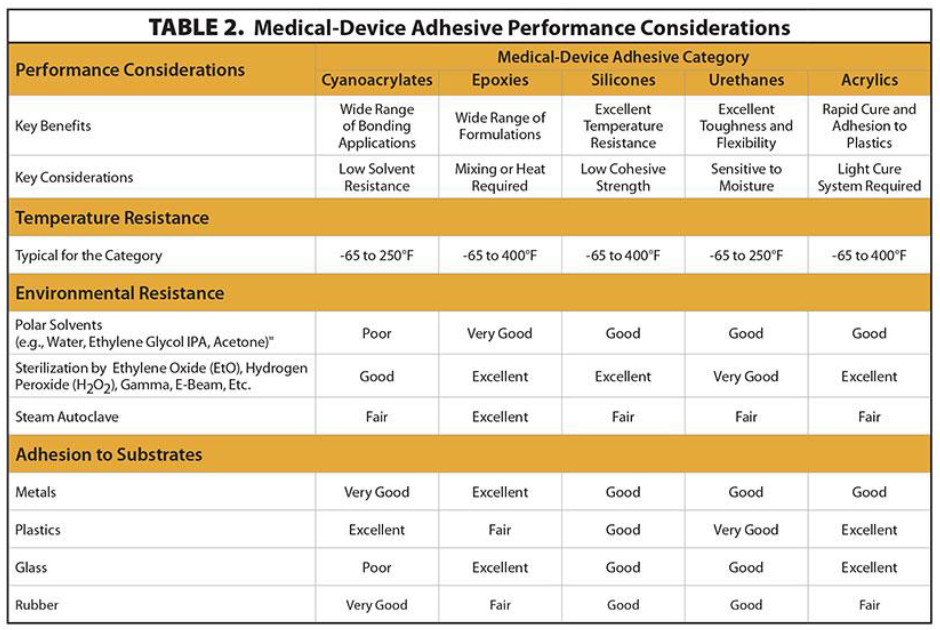
Choosing the Right Oven for Curing Applications
Given the critical role ovens play in thermally curing adhesives, manufacturers must choose the right oven for their production process. Below are some key features to look for in an oven:
Temperature controllers
Manufacturers want a proportional-integral-derivative controller (PID), or three-term controller, in an oven for thermally curing adhesives. PIDs work well in industrial control loop applications. They offer continuously modulated control. Plus, they provide precise temperature control, which medical device manufacturers need. Seek out PIDs with multi-step profile controls, process alarms, data logging, and ease of use.
Heat transfer method
Heat in an oven is transferable using forced convection, natural convection (sometimes called gravity convection), or radiant (infrared) heat sources. Forced convection provides better temperature uniformity than natural convection, especially for high cost of failure medical devices. Radiant heat (infrared) is an efficient way to deliver heat but provides poor temperature control.
Oven atmosphere
Some ovens contain inert atmospheres with nitrogen to stop the oxidation of parts during a soak at elevated temperature. This step involves purging the chamber with nitrogen gas before enabling the heater. Well-sealed ovens have oxygen levels of 10 to 100 ppm.
HEPA filtration
HEPA filtration is critical where foreign matter on a part can result in rejection. Ovens with HEPA-filtered recirculation air can maintain ISO Class 5 cleanroom conditions. But HEPA filters can be less efficient during temperature changes. So, it’s critical manufacturers limit ramp rates on HEPA-filtered ovens to maintain the requisite cleanroom conditions throughout the entire profile.
Oven safety
Hazards associated with adhesives are often involved with the curing process. Ovens should be well ventilated and exhausted to ensure curing by-products don’t impact users. Another related risk is the danger of explosions from solvents in the adhesive formulation. These applications require a Class A oven designed to meet NFPA 86 requirements. While many adhesives lack solvents, some have them. Lesson learned: Know what you’re putting in the oven at all times and the safety measures needed to address their risks.
Curing adhesives is a crucial production step when manufacturing medical devices. While light curing offers expedited curing times and automation benefits, thermal curing remains the preferred method for achieving superior bonding strength and ensuring compliance with stringent industry standards. Industrial ovens can deliver the uniform and repeatable thermal processing needed to boost bonding strength of medical device adhesives. But you need the right oven for the right application along with the right best practices to boost the bonding strength and longevity of adhesives. Failure is not an option when it comes to medical device manufacturing.






